Microglial Cells: Breakthroughs in Alzheimer’s Research
- admin
- 0
- Posted on
Microglial cells, often referred to as the brain’s immune system, play a vital role in maintaining neurological health. These unique cells continuously monitor the brain’s environment, responding to signs of injury, infection, or neurodegenerative disorders such as Alzheimer’s disease. Research led by scientists like Beth Stevens reveals that while microglia are essential for pruning synapses and clearing out dead cells, their malfunction can contribute to the progression of Alzheimer’s disease and other conditions. This transformative understanding has opened doors for identifying potential biomarkers for Alzheimer’s and developing targeted therapies. As millions of Americans grapple with the impact of neurodegenerative diseases, the study of microglial cells is more crucial than ever in advancing our fight against these complex disorders.
The role of glial cells in the brain’s defense mechanisms cannot be overstated, acting as the vigilant guardians within our neurological framework. Known commonly as the brain’s immune cells, these specialized entities are responsible for scanning and responding to changes in the brain, particularly during pathological states like Alzheimer’s disease. Groundbreaking studies from notable researchers, including Beth Stevens, highlight the pivotal function of these cells, emphasizing their contributions to both protective responses and harmful processes when their activity becomes dysregulated. Understanding this duality is essential for developing effective biomarkers for Alzheimer’s and innovative therapeutic approaches. As the quest to combat neurodegenerative disorders continues, insights into these cellular interactions offer hope for future breakthroughs in medical science.
The Role of Microglial Cells in Brain Immunity
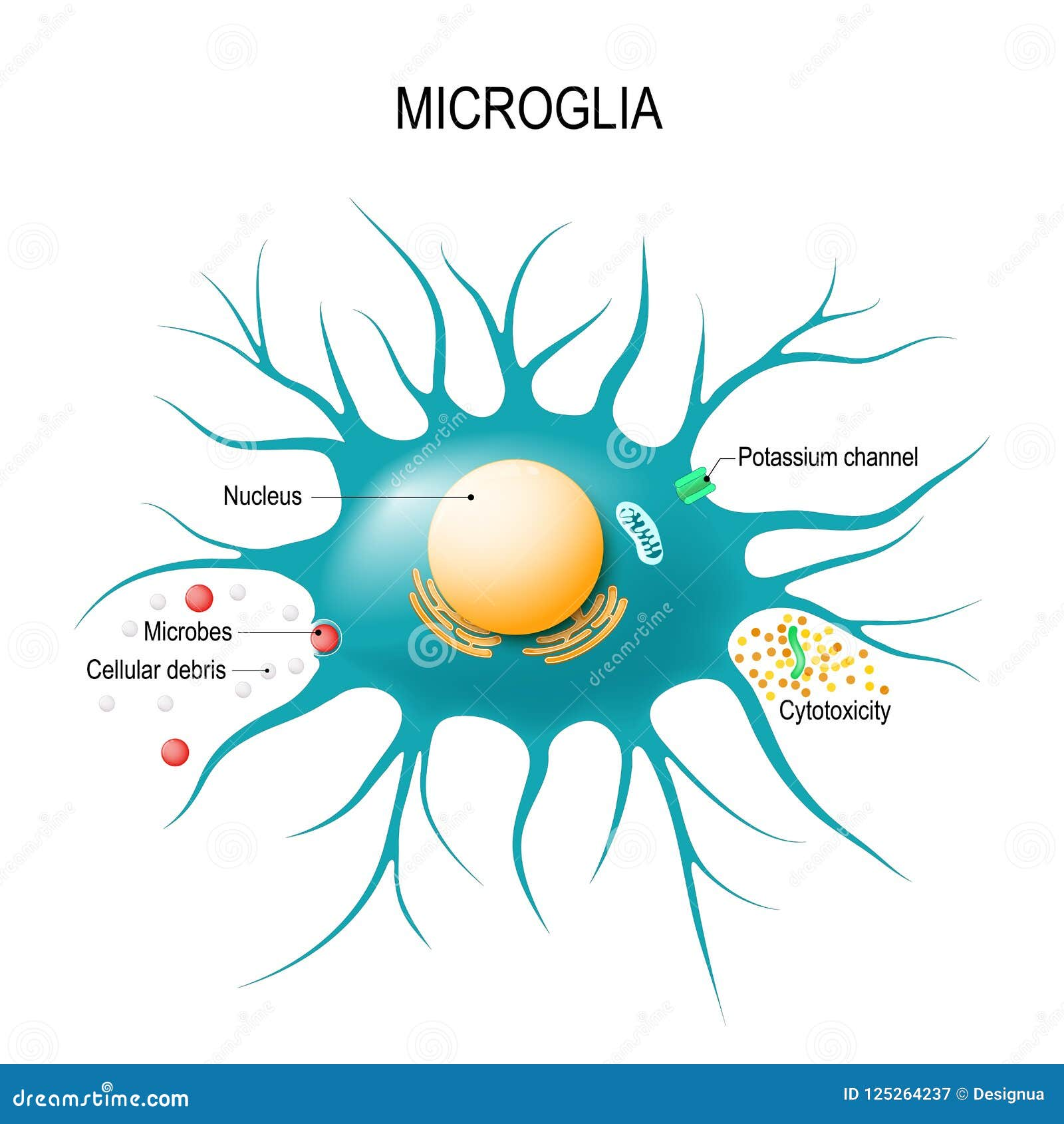
Microglial cells are a critical component of the brain’s immune system, functioning as the first line of defense against various neurological threats, including infections and injuries. These specialized cells constantly survey the microenvironment of the brain and respond to pathological changes, effectively maintaining homeostasis. They help clean up cellular debris, defend against invading pathogens, and modulate neuronal connections through a process known as synaptic pruning. This dynamic role is particularly significant in the context of neurodegenerative disorders, where dysregulation of microglial activity can exacerbate disease progression.
In research conducted by Beth Stevens and her team at Boston Children’s Hospital, it has been revealed that when microglial cells exhibit aberrant pruning behavior, they can inadvertently contribute to conditions like Alzheimer’s disease and Huntington’s disease. This link highlights the dual nature of microglia: while they are essential for brain health, their mismanagement can lead to devastating outcomes. Understanding the mechanisms by which microglia operate opens up new avenues for developing biomarkers for Alzheimer’s and improving therapeutic strategies aimed at enhancing neural health.
Alzheimer’s Disease: Insights from Microglial Research
The connection between microglial functionality and Alzheimer’s disease is an area of intense investigation. Beth Stevens’ research underscores how improper microglial activity can lead to the disease’s hallmark symptoms, including memory loss and cognitive decline. Specifically, her studies have shown that excessive or inappropriate pruning by microglia can deplete synapses crucial for learning and memory, providing insight into the pathology of Alzheimer’s. This highlights the urgent need to explore therapeutic interventions that could stabilize microglial function and prevent neurodegeneration.
Furthermore, Stevens’ ongoing studies aim to identify specific biomarkers for Alzheimer’s disease by examining the behavior of microglial cells in various stages of the illness. By understanding how microglia respond to early pathological changes, researchers hope to develop early detection methods that could significantly improve patient outcomes. This knowledge not only has implications for the millions affected by Alzheimer’s but also sheds light on the broader spectrum of neurodegenerative disorders, with the potential to transform our approach to treatment.
Innovative Biomarkers in Neurodegenerative Disorders
One of the most promising advancements in the realm of Alzheimer’s research is the development of innovative biomarkers that can help identify the disease in its early stages. Through rigorous studies involving the characterization of microglial activity, scientists like Beth Stevens are unraveling the complex interactions within the brain’s immune landscape that contribute to neurodegenerative disorders. These biomarkers can provide vital information about disease progression, enabling clinicians to tailor more effective treatment plans for individuals at risk.
The quest for reliable biomarkers has implications beyond Alzheimer’s disease; it could facilitate early diagnosis and interventions for a wide array of neurodegenerative conditions. By leveraging findings from microglial research, the medical community can pave the way for novel therapeutics designed to harness the natural protective mechanisms of the brain. This commitment to uncovering biomarkers not only enhances our understanding of diseases like Alzheimer’s but also reinforces the importance of continued research into the brain’s immune system as a means to combat neurological deterioration.
The Legacy and Future of Microglial Research
Beth Stevens’ transformative work on microglial cells has redefined our understanding of the brain’s immune system and its implications for neurodegenerative diseases. Her journey from basic science to translational research reflects the profound impact that fundamental discoveries can have on clinical practice. With a clear focus on how microglial cells interact with neural circuits, Stevens’ research has illuminated the pathway from curiosity-driven science to tangible advancements in treating Alzheimer’s disease and other conditions. This legacy emphasizes the necessity of sustained funding for basic research, as it serves as the backbone for future scientific breakthroughs.
Looking ahead, the future of microglial research promises to unveil even more critical insights into brain immunity and neurodegeneration. As we develop a deeper understanding of microglial function, researchers are optimistic about the potential for creating targeted therapies that not only slow down the progression of Alzheimer’s disease but also restore brain health. The integration of technologies such as imaging and gene editing may further accelerate the discovery of novel biomarkers and therapeutic approaches, ultimately advancing our ability to combat the challenges posed by neurodegenerative disorders.
Challenges in Understanding Neurodegenerative Disorders
Despite significant advancements in neuroscience, understanding the complexities of neurodegenerative disorders remains a daunting challenge. Diseases like Alzheimer’s are not only characterized by toxic protein accumulation but also involve immune system dysfunction, particularly the role of microglial cells. Researchers continue to grapple with how to translate findings from laboratory settings to clinical outcomes, which underscores the need for comprehensive studies that encompass both animal models and human data. This multifaceted approach is crucial for elucidating the interplay between microglial activity and the pathogenesis of neurodegenerative diseases.
Additionally, there exists a variety of genetic and environmental factors that contribute to the onset of these disorders, complicating the search for universal solutions. The insights gained from Beth Stevens’ research highlight the need for personalized medicine approaches that consider individual variability in microglial responses and cognitive functions. As the scientific community pushes forward, the integration of interdisciplinary perspectives will be essential to unlocking mechanisms of disease and developing accessible treatments for those afflicted with neurodegenerative disorders like Alzheimer’s.
Federal Funding and Support for Neuroscience Research
Federal funding plays a critical role in advancing neuroscience research, particularly in the fight against Alzheimer’s disease. Investigators like Beth Stevens emphasize that without support from agencies like the National Institutes of Health (NIH), much of the groundbreaking work focusing on microglial research would not be possible. These funds not only facilitate basic science but also enable researchers to explore innovative ideas that could lead to significant medical advancements. This underscores the importance of advocacy for sustained funding to ensure continuous progress in understanding and treating neurodegenerative disorders.
Moreover, the support from federal sources fosters an environment where scientific curiosity can thrive, leading to unexpected discoveries that can cascade into transformative outcomes. Stevens’ journey exemplifies this, highlighting that the initial study of microglial cells, focused on their fundamental role in brain health, has spearheaded new pathways for disease detection and therapeutic intervention. Continued investment in neuroscience research is crucial for empowering scientists to chase curiosity-driven questions, ultimately culminating in impactful solutions for diseases such as Alzheimer’s.
The Interconnection of Basic Science and Clinical Outcomes
The relationship between basic science and clinical outcomes is a foundational aspect of medical research, particularly in the field of neurodegenerative disorders like Alzheimer’s disease. Research conducted by scientists such as Beth Stevens illustrates how early studies on microglial cells can lead to breakthroughs in understanding and treating complex conditions. This interplay highlights the significance of foundational research as it forms the groundwork for clinical innovations, potentially improving the quality of life for millions affected by Alzheimer’s.
Furthermore, the path from basic science to clinical application often requires a multidisciplinary approach, incorporating insights from genetics, immunology, and psychology. This comprehensive strategy allows researchers to draw connections between microglial activity and cognitive decline, creating an integrated framework for developing effective treatments. As the scientific community continues to support this interconnection, the potential for impactful advancements in the field becomes more promising, further reinforcing the value of curiosity-driven research in the realm of neuroscience.
Public Awareness and Education on Alzheimer’s Disease
Raising public awareness and education about Alzheimer’s disease is essential for fostering understanding and support for research initiatives. Educating the community about the role of microglial cells and their connection to neurodegenerative disorders can empower individuals and families affected by the disease. Initiatives led by researchers like Beth Stevens not only enhance public knowledge but also encourage advocacy for funding and research support, paving the way for new advancements in Alzheimer’s treatment and detection.
Furthermore, increasing awareness about the importance of brain health can promote preventive measures that may help minimize the risk of developing Alzheimer’s disease. By elucidating the role of the brain’s immune system and microglial cells in maintaining neural health, individuals can be encouraged to adopt healthier lifestyles and participate in regular cognitive activities, contributing to overall brain well-being. Initiatives that prioritize public education are vital for creating a supportive environment for ongoing research and gathering momentum in the quest for a cure.
The Importance of Collaborative Research in Neuroscience
Collaborative research efforts have proven to be pivotal in advancing the field of neuroscience, particularly in addressing complex challenges posed by neurodegenerative disorders. The interplay between various research institutions, like the Broad Institute and Boston Children’s Hospital, amplifies the potential for discoveries that might not be achievable in solitary settings. Beth Stevens exemplifies this collaborative spirit through her partnerships that bring together diverse expertise to focus on microglial function and its implications for diseases such as Alzheimer’s.
Such interdisciplinary collaborations not only promote shared knowledge but also foster innovation by integrating diverse methodologies and perspectives. As researchers work together, they can tackle multifaceted problems and approach research questions from multiple angles, leading to richer insights into the mechanisms underlying neurodegeneration. By continuing to nurture and expand collaborative initiatives in neuroscience research, we can accelerate the pace of innovation and ultimately improve treatment approaches for Alzheimer’s and other neurodegenerative disorders.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s disease?
Microglial cells act as the brain’s immune system, monitoring for signs of illness or injury. In Alzheimer’s disease, they are crucial for clearing away dead or damaged cells. However, improper functioning of these cells can lead to aberrant pruning of synapses, potentially exacerbating neurodegenerative processes associated with Alzheimer’s.
How do microglial cells contribute to the brain’s immune response?
Microglial cells are essential components of the brain’s immune system. They constantly survey the brain environment for pathogens or distressed cells, engaging in processes like phagocytosis to remove debris and support neuronal health. Their dysfunction is linked to various neurodegenerative disorders, including Alzheimer’s disease.
What discoveries has Beth Stevens made regarding microglial cells and neurodegenerative disorders?
Beth Stevens has shown that microglial cells are involved in synaptic pruning and that this process can go awry in diseases like Alzheimer’s and Huntington’s. Her research has provided insights into the potential for new biomarkers and therapies to address these neurodegenerative disorders.
Can microglial cells be targeted for Alzheimer’s disease treatment?
Yes, targeting microglial cells is an emerging strategy in Alzheimer’s disease treatment. Investigations into their role in synaptic pruning and inflammation are paving the way for potential biomarkers and therapeutic interventions aimed at enhancing their function or correcting their dysfunction.
What are the potential biomarkers for Alzheimer’s related to microglial activity?
Research led by scientists like Beth Stevens is identifying potential biomarkers linked to microglial activity in Alzheimer’s disease. These biomarkers could help detect aberrant microglial function or inflammation earlier, providing critical insights into the disease’s progression and effectiveness of treatments.
How does microglial dysfunction relate to other neurodegenerative disorders?
Dysfunctional microglial cells are implicated not only in Alzheimer’s disease but also in other neurodegenerative disorders such as Huntington’s disease. Their role in orchestrating the brain’s immune response and promoting neuroprotection or neuroinflammation can significantly influence disease outcomes.
What is the connection between microglial cells and synaptic pruning in Alzheimer’s?
Microglial cells are responsible for synaptic pruning during brain development and in response to injury. In Alzheimer’s disease, abnormal synaptic pruning by microglia can lead to loss of communication between neurons, contributing to cognitive decline and the progression of neurodegenerative changes.
Why are microglial cells important for research in Alzheimer’s disease?
Research on microglial cells is vital for understanding Alzheimer’s disease mechanisms. These immune cells provide insights into how the brain responds to damage and how aberrant behaviors can lead to neurodegeneration. This knowledge is crucial for developing effective therapies and interventions.
| Key Point | Details |
|---|---|
| Microglial Cells | Act as the brain’s immune system, patrolling for illness and injury. |
| Role in Synaptic Pruning | Help clear dead cells and prune synapses, essential for brain health and development. |
| Connection to Disease | Aberrant pruning linked to Alzheimer’s, Huntington’s disease, and others. |
| Research Impact | Findings support development of new biomarkers and treatments for neurodegenerative diseases. |
| Funding Importance | Research foundationally supported by NIH and other federal agencies, crucial for breakthroughs. |
Summary
Microglial cells play a crucial role in the brain’s immune defense and maintaining synaptic health. As described by researcher Beth Stevens, they are vital for identifying and eliminating damaged cells, helping to protect against neurodegenerative diseases like Alzheimer’s. Understanding the function and dysfunction of microglial cells is essential for developing effective treatments and interventions for millions affected by such conditions.